Energy Storage Materials
The Moore group is actively contributing to the development of materials for the next generation energy storage systems. Our main projects are the preparation and study of new redox active molecules, electrolytes for non-aqueous media, and polymeric membranes and separators.
Electrochemically reversible fluids of high energy density are promising materials for capturing the electrical energy generated from intermittent sources like solar and wind. To meet this technological challenge there is a need to understand the fundamental chemistry and transport properties of redox-active molecules and macromolecules. Our research builds on the group’s expertise in organic, polymer, and colloidal particle synthesis, as well as physical organic chemistry. We are developing redox-active polymers (RAPs) and redox-active colloids (RACs) to overcome limitations of battery electrolytes for grid storage. The project is highly collaborative and is part of the Joint Center for Energy Storage Research, also known as JCESR. Moore group members collaborate closely with the Rodriguez-Lopez group, among others.
Size-Exclusion Strategy for Active Material Crossover Prevention
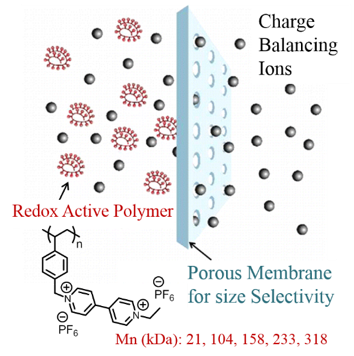 Non-aqueous flow batteries (NRFB) offer high energy density compared to aqueous flow batteries. However, NRFBs suffer from active material crossover and low ionic conductivity, which are the major hurdles to be overcome for their commercialization. Thus, fast and selective transport of the charge balancing ions and negligible crossover of the redox active material are highly desired. We have provided proof of concept demonstration for redox active viologen polymers in which Coulombic efficiency of flowable electrodes is maintained by size-exclusion based mechanism of separation. The polymer materials exhibit high solubility in propylene carbonate (one molar) to permit high storage capacity. Size-exclusion strategy will enable the use of commercial off-the-shelf (COTS) membranes in flow batteries, thus helps to bring down the cost, and increase the ionic conductivity and Coulombic efficiency of flow batteries. For the first time a redox active polymer that meets the requirements for use as anolyte in NRFBs is developed. Molecular design principles here are useful to develop redox active nanostructures and colloidal particles for use in non-aqueous colloidal flow batteries.
Non-aqueous flow batteries (NRFB) offer high energy density compared to aqueous flow batteries. However, NRFBs suffer from active material crossover and low ionic conductivity, which are the major hurdles to be overcome for their commercialization. Thus, fast and selective transport of the charge balancing ions and negligible crossover of the redox active material are highly desired. We have provided proof of concept demonstration for redox active viologen polymers in which Coulombic efficiency of flowable electrodes is maintained by size-exclusion based mechanism of separation. The polymer materials exhibit high solubility in propylene carbonate (one molar) to permit high storage capacity. Size-exclusion strategy will enable the use of commercial off-the-shelf (COTS) membranes in flow batteries, thus helps to bring down the cost, and increase the ionic conductivity and Coulombic efficiency of flow batteries. For the first time a redox active polymer that meets the requirements for use as anolyte in NRFBs is developed. Molecular design principles here are useful to develop redox active nanostructures and colloidal particles for use in non-aqueous colloidal flow batteries.
Polymer Design for Superior Electrochemical Performance
The design of chemically stable and electrochemically reversible 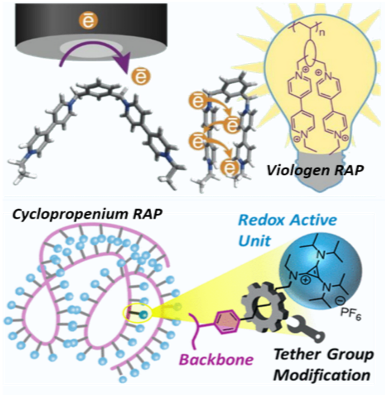 RAPs is of great interest for energy storage technologies. The tether length and position of pendants have a pronounced role on their reactivity, electrochemical stability and long-distance charge transfer kinetics. We have used systematic molecular design approaches to investigate the impact of pendant-backbone electronic interactions on the performance of redox-active pendant units. Our study of viologen oligomers and polymers revealed that a shorter or more rigid tether have positive shifts in the reduction potential and exhibit enhanced levels of electrochemical kinetics. The investigation of cyclopropenium appended polymers indicated that polymers with extended tether groups display an improved reversibility in cyclic voltammetry. The behavior is mirrored in the stability of the charged state tested in galvanostatic half-cells. The capacity decays for the polymers were structure-dependent, providing empirical insight into the design of next generation of RAP materials.
RAPs is of great interest for energy storage technologies. The tether length and position of pendants have a pronounced role on their reactivity, electrochemical stability and long-distance charge transfer kinetics. We have used systematic molecular design approaches to investigate the impact of pendant-backbone electronic interactions on the performance of redox-active pendant units. Our study of viologen oligomers and polymers revealed that a shorter or more rigid tether have positive shifts in the reduction potential and exhibit enhanced levels of electrochemical kinetics. The investigation of cyclopropenium appended polymers indicated that polymers with extended tether groups display an improved reversibility in cyclic voltammetry. The behavior is mirrored in the stability of the charged state tested in galvanostatic half-cells. The capacity decays for the polymers were structure-dependent, providing empirical insight into the design of next generation of RAP materials.
